Clinical trials speed up because of COVID
(NEW BRUNSWICK) – The coronavirus pandemic has caused untold misery, pain and suffering, but it has also revolutionized how clinical trials for new drugs and treatments are carried out.
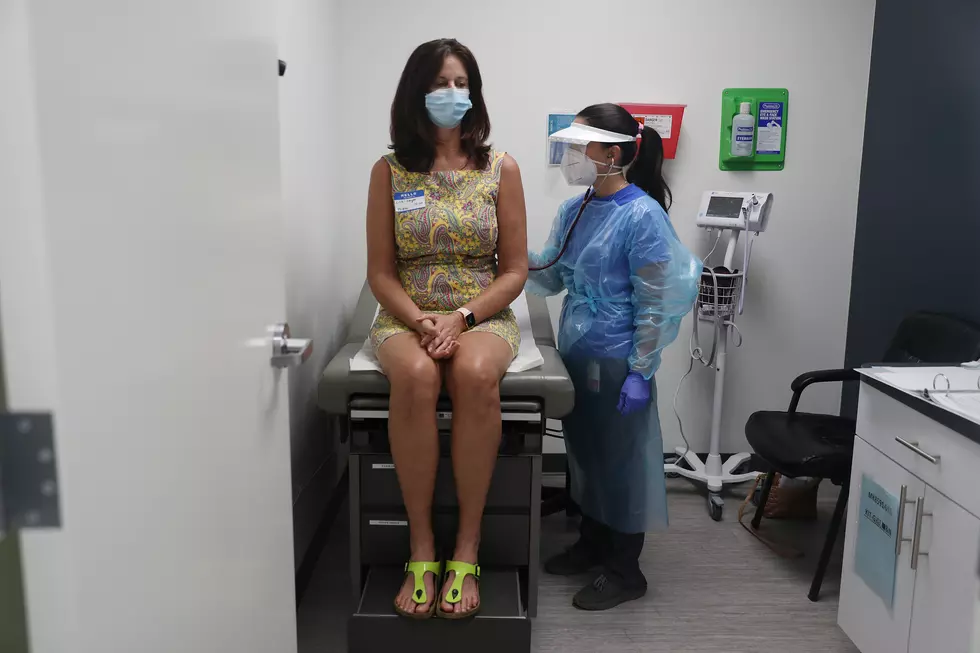
When the pandemic first started in March 2020, clinical trials – like nearly everything else in New Jersey – came to a grinding halt as people were told to stay home. With work still needing to continue, clinical trials were forced to abruptly shift gears.
Prior to the pandemic, clinical trials were run out of clinical research units at medical schools, doctors offices and other in-person locations, which involved participants traveling to brick-and-mortar study sites to be evaluated, said Dr. Reynold Panettieri, vice chancellor for translational medicine and science and director of the Rutgers University Institute for Translational Medicine.
That structure had to change once the pandemic hit.
Clinical trials go virtual
When the COVID emergency was declared, a virtual approach was adopted.
Panettieri said traditional clinical trials typically took six months to implement and a year to fully recruit participants, but this new online electronic approach decreased implementation and recruitment times by 50%.
“We’re reaching out to people in homes. They don’t have to travel. I think there’s greater availability for the underserved populations in getting into clinical trials,” he said.
Panettieri said this new approach is transformative and will enhance “the nimbleness of doing these trials, getting the data faster to the FDA, and hopefully decrease the length of time necessary to get drugs approved.”
Costs lowered in virtual clinical trials
Another benefit to conducting trials virtually – the costs are less.
Panettieri said conducting virtual clinical trials, which includes remote data collection and telemedicine consultations, will lower costs for pharmaceutical companies.
It's a benefit that patients will see as well.
“Patients are going to be benefiting because if the drugs can be approved in a safe and effective manner, it’s going to get those drugs to the providers to give them to the patients faster,” Panettieri said.
He said this new approach will also help to decrease staff needs for those organizations conducting the clinical trials.
Panettieri said the bottom line is having the capability of decentralized clinical trials “will offer a more contemporary approach, meeting the needs of participants, sponsors, the government, and academic medical centers as partners in the development of new drugs."
Clinical trials currently at Rutgers
According to a press release from Rutgers, here are some examples of virtual clinical trials currently being conducted at the university now:
- A study examining if home air cleaners reduce the level of coronavirus in homes of COVID-19 positive adults. Researchers were able to expand the number of participants from 20 to 40 thanks to the virtual approach.
- A study that tests the preliminary efficacy of two doses of a combination of probiotics given to boost the immunity of unvaccinated people with prior confirmed SARS-CoV-2 infection. In partnership with Vault Medical Services, Rutgers was able to recruit participants in five states to conduct at-home visits.
- NJ HEROES (New Jersey Healthcare Essential Worker Outreach and Education Study - Testing Overlooked Occupations) is a clinical study that aims to better understand COVID-19 testing patterns among underserved and vulnerable populations so that strategies can be developed to reduce disparities in COVID-19 testing. Rutgers partnered with Vault Medical Services to provide remote testing for 1,963 participants.
You can contact reporter David Matthau at david.matthau@townsquaremedia.com.
What to know about the spotted lanternfly & tree of heaven in NJ
Incredible, heartbreaking images of Ida's damage in New Jersey
More From Beach Radio










